Our lab investigates the interactions between macrophages and other cell types. Macrophages are regulatory cells that can function in a wide range of activities, ranging from killing bacteria and tumor cells to healing wounds and promoting angiogenesis, and their plasticity is determined by the changing microenvironment and signals emitted from the tissue cells.
CD147/EMMPRIN as a regulator of macrophage interactions:
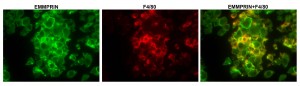
Tumor cells actively recruit macrophages into the tumor mass, and reprogram them to secrete pro-angiogenic and pro-metastatic factors that are necessary for tumor growth and metastasis (e.g., VEGF and MMPs). CD147/EMMPRIN is gaining recognition as a protein that mediates such interactions, which is overexpressed on many types of tumor cells, and induces macrophages to secrete high amounts of VEGF and MMPs that promote angiogenesis and metastasis. EMMPRIN expression is also elevated in inflammatory epithelial cells and plays a role in angiogenesis in autoimmune diseases, such as Rheumatoid Arthritis (RA), Psoriatic arthritis (PsA) and type 2 Diabetes Mellitus (T2DM).
We currently explore:
- Targeting a novel epitope in the EMMPRIN protein by either a monoclonal antibody or by active peptide vaccination.
- Signaling pathways that regulate EMMPRIN expression and activity.
- The role EMMPRIN plays in the regulation of metastasis and dormancy
- EMMPRIN as regulator of angiogenesis in autoimmune diseases (RA, PsA, T2DM).
EMMPRIN as a regulator of angiogenesis in autoimmune diseases:
Angiogenesis is critical in chronic inflammatory diseases, and ensures the constant supply of nutrients and oxygen to the immune infiltrate. However, pathological angiogenesis could be involved in clinical complications (e.g., retinopathy, nephropathy).
We currently explore:
- The regulatory effects of EMMPRIN on angiogenesis in RA and T2DM
- Effects of treatment (e.g., disease-modifying anti-rheumatic drugs (DMARDs) or metformin) on pro-angiogenic factors.